Woman almost dies after taking cough medicine: "I flatlined completely"

It’s the over-the-counter product that so many of have taken to help alleviate a cold. But for some, the combination of cough syrups and aesthetics can be potentially fatal.
After Australian woman Narelle Campbell, 52, was given a general anaesthetic during an operation for an aneurism, she had a catastrophic allergic reaction after taking a cough medicine prior, reports 9News. Doctors had to break four ribs to bring her back to life.
“I flatlined completely – I was gone,” Campbell said in an interview with 9News.
“The thing that surprises me is I went in there to have an operation for an aneurism and I actually died on the table because of cough medicine.”
It happened because of the active ingredient pholcodine, an antitussive or cough-suppressing compound which NPS Medicine Wise says has been used in cough medicines since the 1950s. It is banned in the US but alarmingly, it can be found in over 50 products in Australia, including household names like Benadryl, Difflam and Duro-Truss.
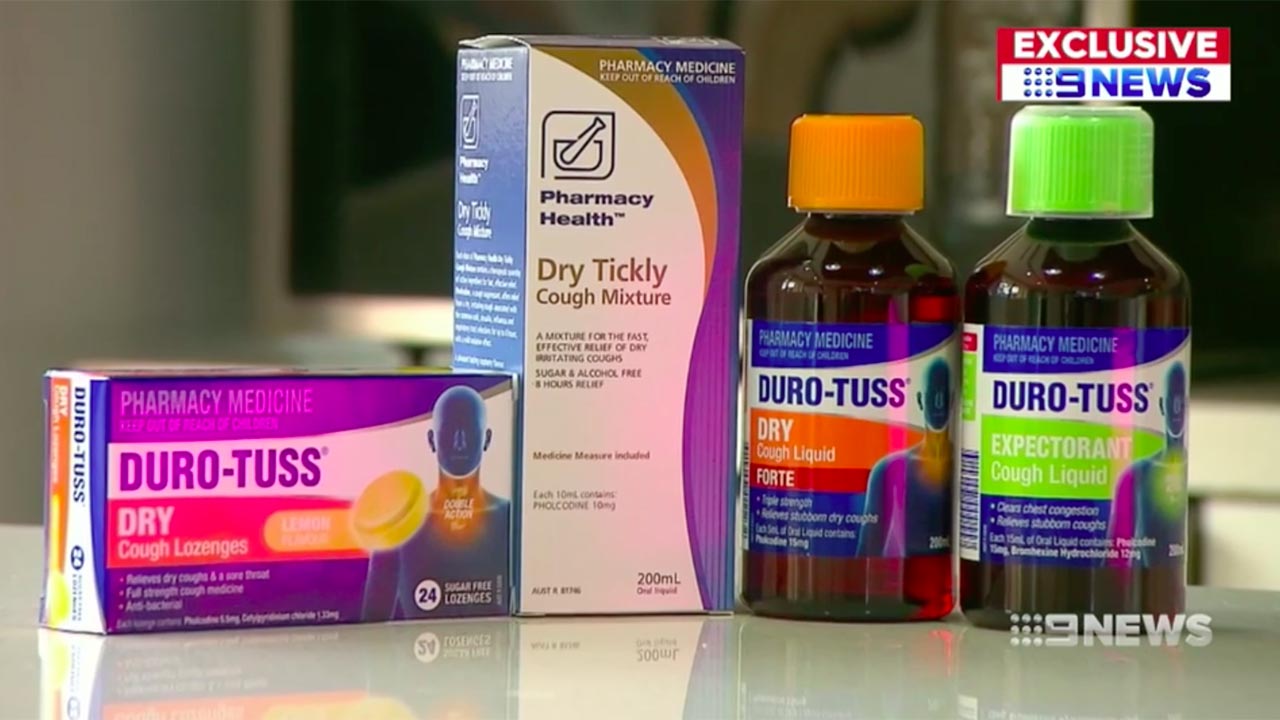
In 2015, Dr Michael Rose, Chairman of the Australian and New Zealand Anaesthetic Allergy Group (ANZAAG), told ABC NEWS that the group was calling for products with the chemical to be made prescription-only.
He said that some studies have found that pholcodine could create an antibody in a small proportion of people that causes an allergic reaction to some anaesthetics because of the muscle relaxants contained in them.
Dr Paul McAleer of ANZAAG told 9News that surgical anaphylaxis during anaesthesia causes over seven deaths every three years.
“We believe pholcodine plays a part in de-sensitising some people to the effects of muscle relaxants in anaesthesia,” he said.
Despite the deaths, the Therapeutic Goods Administration (TGA) believes there isn’t sufficient evidence to ban the chemical, after anaesthetists made the case to have cough medicines with pholcodine made prescription-only or banned altogether, in submissions in 2013 and 2015. After this latest case, they continue to lobby for tighter restrictions.
The most compelling case for banning the drug was in Scandinavia, according to ABC News, with the startling contrast between surgical anaphylaxis between Norway and Sweden. Norway’s rate a decade ago was 10 times higher than Sweden’s. The use of pholcodine was high in Norway, but in Sweden it was banned.
"In 2007 the drug company that was manufacturing pholcodine in Norway voluntarily removed it from the market," explained Dr Rose.
"And since that time the rate of allergic reactions to muscle relaxants has fallen and the rate of antibodies in the population has also fallen."
Dr McAleer advises consumers to avoid products with pholcodine if they can.
And if you’re having surgery, be sure to let the anaesthetist overseeing you know if you’ve had any antitussives leading up to admission to hospital.
Do you have any of these cough syrups in your medicine cabinet at home? Tell us in the comments below.
